Article Text
Abstract
Background: Adult normal pressure hydrocephalus (NPH) is one of the few potentially treatable causes of dementia. Some morphological and functional abnormalities attributed to hydrocephalus improve following treatment.
Objectives: We focused on analysis of changes in cerebral metabolites using proton magnetic resonance spectroscopy (1H-MRS) after NPH treatment, and its clinical and cognitive correlation.
Methods:1H-MRS, neuropsychological and clinical status examinations were performed before and 6 months after shunting in 12 adults with idiopathic NPH. We obtained N-acetyl-aspartate (NAA), choline (Cho), myoinositol (MI) and creatine (Cr) values.
Results: After surgery, NAA/Cr was significantly increased. Moreover, NAA/Cr values were related to cognitive deterioration.
Conclusion: MRS could be a marker of neuronal dysfunction in NPH.
- Cr, creatine
- Cho, choline
- 1H-MRS, proton magnetic resonance spectroscopy
- MI, myoinositol
- MMSE, Mini-Mental State Examination
- NAA, N-acetyl-aspartate
- NPH, normal pressure hydrocephalus
Statistics from Altmetric.com
- Cr, creatine
- Cho, choline
- 1H-MRS, proton magnetic resonance spectroscopy
- MI, myoinositol
- MMSE, Mini-Mental State Examination
- NAA, N-acetyl-aspartate
- NPH, normal pressure hydrocephalus
Normal pressure hydrocephalus (NPH) is a potentially treatable cause of dementia,1,2 characterised by progressive cognitive dysfunction, gait disturbance and urinary incontinence associated with ventricular enlargement and abnormalities in CSF dynamics. In these patients, some morphological and functional abnormalities attributed to hydrocephalus improve after treatment.3–5 Proton magnetic resonance spectroscopy (1H-MRS) allows non-invasive in vivo measurement of brain metabolites. Findings from MRS studies reveal that 1H-MRS is a potentially non-invasive technique with sufficient sensitivity to detect subtle changes in neuronal function in neurodegenerative diseases, allowing investigation of neuronal injury or dysfunction6,7 and the assessment of treatment efficacy.8,9,10
1H-MRS studies in patients with hydrocephalus are scarce.6,7,11–15 Changes in cerebral metabolites after treatment with hydrocephalus using this technique have been analysed in only two studies, which concentrated exclusively on the results of lactate metabolites.11,12
The aim of our study was to describe changes in other major metabolites, using 1H-MRS, before and after treatment in idiopathic NPH patients, and to obtain preliminary data on their clinical and cognitive correlation, which could serve as the basis for larger studies with control subjects.
METHODS
Subjects
Twelve patients with idiopathic NPH, evaluated between March 2001 and July 2002, were included in the study (six women and six men, 72.08 (7.43) years old, 7.33 (7.44) years of education). Tables 1 and 2 show the clinical and therapeutic data for these patients.
Clinical data
Metabolic and neuropsychological changes after treatment
Patients were selected for shunting according to our NPH management protocol.16 The shunt materials were compatible with MRI and did not produce artefacts in the examination. The ventriculostomy performed during shunt implantation did not distort the cerebral tissue included in the voxel selected for the study.
All patients underwent neurological, combined MRI/1H-MRS and neuropsychological examinations, both before and 6 months after surgery. Postoperative N-acetyl-aspartate (NAA) values were not obtained in one patient.
MRI and spectroscopy
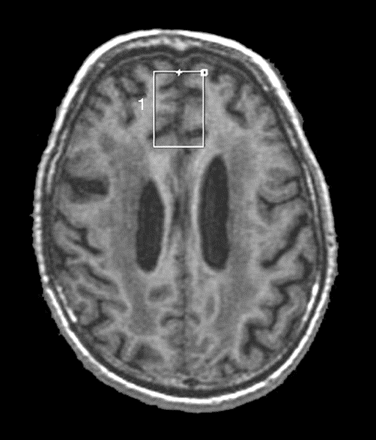
1H-MRS was performed using a 1.5 T whole body MR scanner (General Electric Signa System, Milwaukee, Wisconsin, USA) with a head coil. Proton spectra were obtained from a single voxel in an axial image. In all subjects, the voxel was placed in the medial frontal regions, covering the anterior cingulate in both hemispheres (fig 1).
Axial image showing placement of the voxel of interest during proton magnetic resonance spectroscopy (1H-MRS) of a patient. To achieve a reproducible position, the voxels were placed in the same regions in all patients. Half of the voxel was placed in the interhemispheric fissure. The area covered by the voxel corresponded to the superior frontal girus, the cingular sulci and the more anterior side of the anterior cingular girus. The posterior boundary of the voxel was placed on the reference slice immediately adjacent to the anterior margin of the genu of the corpus callosum. The voxel was placed in both the right and left frontal lobes.
Water suppressed spectra were acquired using a double spin echo, point resolved spectroscopy sequence with a repetition time of 1500 ms and an echo time of 35 ms for the medial frontal lobe voxel. The spectra were analysed using the MRS software package supplied by the manufacturer. NAA, choline (Cho), myoinositol (MI) and creatine (Cr) values were obtained. SPM5 segmentation was performed to correct for the amount of grey matter, white matter and CSF in the voxel of interest.
Neuropsychological and clinical assessment
Neuropsychological testing was performed before and 6 months after surgery. All patients were evaluated with cognitive tests, specifically selected to cover memory and frontal functions, and with a brief screening test for dementia (Mini-Mental State Examination (MMSE)) (table 1).
We used information and similarities subtest scores from the Wechsler adult intelligence scale as an indicator of the pre-morbid intelligence quotient. Details of all the tests are described by Lezak et al.17
The NPH Scale18 was used to evaluate the three main components of the NPH syndrome: gait, and cognitive and sphincter disturbances.
Statistical analysis
All statistical analyses were performed using SPSS 11.0 for Windows (SPSS Inc., Chicago, Illinois, USA). Normally distributed data assumption was tested using the Komolgorov–Smirnov test. Comparisons among clinical and neuropsychological data were made using the t test for paired samples. 1H-MRS values before and after surgery were compared using the general linear model of repeated measures taking as a covariant the percentage of change in grey matter, white matter and CSF in the voxel of interest. We calculated percentage of change as follows: ((postoperative − preoperative)/preoperative) ×100.4,5 Partial correlation controlling for the amount of grey matter, white matter and CSF in the voxel of interest was used to relate the 1H-MRS metabolite peak ratios with neuropsychological performance, clinical status and age before and after surgery. The relation between the percentage change in 1H-MRS values and percentage of cognitive and motor function changes was also analysed using partial correlation. Statistical significance was considered when p<0.01.
RESULTS
Eleven patients improved, showing an increase of 1 or more points on the NPH Scale. No change on the NPH Scale was found in the remaining patient (pre- and post-surgical scores = 13). This patient, however, showed functional improvement in daily activities. There was no treatment related mortality. A small asymptomatic hygroma was diagnosed in one patient 6 months after shunting but this did not require treatment. These results coincide with data from other series analysed in our department.16
Tables 1 and 2 summarise the neuropsychological and clinical data of the patients. Comparisons between preoperative and postoperative cognitive and clinical data showed a statistically significant improvement in 7 of the 16 tests or scales used: gait (t = −4.750, p = 0.005), sphincter control (t = 3.761, p = 0.003), cognitive performance (t = 4.168, p = 0.002), general NPH score (t = 5.422, p<0.001), long term retention variables on the Rey Auditory Verbal Learning Test (t = −3.268, p = 0.007), visual reproduction (subtest of the Wechsler Memory Scale-Revised: t = −3.669, p = 0.004) and backward digit span (t = −3.924, p = 0.008).
NAA/Cr and NAA/Cho mean values increased after surgery and Cho/Cr and MI/Cr mean values decreased (table 2). Statistical comparisons between preoperative and postoperative metabolite ratios, using a within subject design with covariables, showed a significant increase in NAA/Cr (F = 13.168, p = 0.008).
Before surgery, there was a tendency towards a significant relationship between the preoperative frontal NAA/Cr ratio with learning of the Rey Auditory Verbal Learning Test (Pearson’s r = 0.737, p = 0.015) and NAA/Cr ratio with working memory assessed by means of backward digit span (Pearson’s r = 0.760, p = 0.011). After surgery, the postoperative frontal NAA/Cr ratio was found to be related to the postoperative MMSE score (Pearson’s r = 0.856, p = 0.003). Only two patients did not show an increase in the NAA/Cr ratio after surgery. In one of these patients, the MMSE score decreased.
Age, pre-morbid intelligence quotient and clinical status were not related to NAA/Cr metabolite peak ratios before or after surgery.
No association was found between changes in NAA/Cr values and clinical or neuropsychological changes.
DISCUSSION
To our knowledge, this is the first report of the effects of surgery on cerebral metabolism in adults with idiopathic NPH using non-invasive methods. Reduction of several neurotransmitters and neuropeptides in the CSF of hydrocephalic patients has been described by several authors.5,19 Previous studies performed in our department in patients with NPH showed that levels of somatostatin, neuropeptide Y, corticotropin releasing factor and galanin change significantly after shunting in lumbar CSF.4,5 Importantly, galanin reduction after shunting correlated significantly with cognitive improvement in the patients studied.4 However, these tests required invasive manoeuvres and, in patients with altered CSF volume and dynamics, the real significance of these findings is still debated.
Our main results showed a significant increase in NAA/Cr after operation. Partial recovery of decreases in NAA have been observed after treatment in several pathological conditions, such as schizophrenia,20 amyotrophic lateral sclerosis,9 multiple sclerosis10 and AIDS associated cognitive impairment.21 NAA is present primarily in neurons, axons and dendrites, and is viewed as an indicator of neuronal state and function. NAA is also important for reparative brain processes by enhancing lipid synthesis and repairing injured myelin.22 Hydrocephalus is associated with atrophy and gliosis of periventricular white matter and damage to myelin production,23 which could explain the NAA decrease in these patients.
Given that clinical and neuropsychological research indicates that the frontal lobe is involved in the pathophysiology of NPH, we decided to place our voxel on the prefrontal region. However, studies with voxels localised in other brain regions could be useful in determining whether 1H-MRS metabolite changes in patients with NPH are region specific.
Studies performed in human hydrocephalus have described some reversal of functional changes after shunting, some of these changes being related to frontal areas.3,4,24 In our patients, all assessed clinical and neuropsychological functions improved after surgery, with statistically significant increases in NPH Scale scores, memory (visual immediate memory, verbal long term memory) and working memory as a frontal function. These results confirm and expand on previous NPH studies that have found a significant improvement in clinical and fronto-subcortical functions after shunting.3,4
We found a tendency towards a correlation between preoperative frontal NAA/Cr ratios and some cognitive functions before treatment. The NAA/Cr ratio was related to verbal learning. Impaired retrieval efficiency can be interpreted as reflecting frontal lobe dysfunction, given that recall requires strategic and exertive retrieval, which is mediated by the frontal lobes.25 Moreover, the NAA/Cr ratio was related to working memory. Some studies have reported a relationship between NAA frontal levels and frontal functions, such as working memory, and executive and attention level functions.8,26 After surgery, postoperative frontal NAA/Cr ratios correlated significantly with measurement of global cognitive impairment. This correlation is reasonable given that the MMSE is a screening test designed to evaluate the brain as a whole, and improvement in frontal function, such as attention, concentration or executive function, could result in an improvement in global cognitive performance.
CONCLUSIONS
Our results show that NAA/Cr significantly increases after surgical treatment of hydrocephalus. NAA/Cr values correlated with a global index of cognitive impairment. NAA in the medial frontal cortex could be a dynamic marker of neuronal function in patients with idiopathic NPH. Further studies with larger samples and a control group with repeated measures are needed to corroborate these findings.
Acknowledgments
This study was supported by the following grants: Red Temática de Investigación Cooperativa de Enfermedades Neurológicas (RED CIEN) of the Fondo de Investigaciones Sanitarias (C3/06), and 2001SGR00139 and 2005SGR00411 (Generalitat de Catalunya). María del Mar Matarín was funded by a pre-doctoral grant from the University of Barcelona.
REFERENCES
Footnotes
-
Published Online First 13 February 2007
-
Competing interests: None.
-
The protocol of the study was approved by the Institutional Ethical Committee on Human Research of Vall d’Hebron University Hospital (PR [HG] 63/2001). Consent was obtained from patients’ next of kin after the nature of the procedure had been fully explained.